Abstract
Introduction
Asparaginase is incorporated into many multi-agent chemotherapy regimens used in the treatment of acute lymphoblastic leukemia (ALL). Asparaginase depletes proteins including stores of factors in the coagulation cascade and proteins regulating it, including antithrombin III (AT) and fibrinogen. This results in an increased risk of both bleeding and thrombosis. The rate of thrombosis following administration of asparaginase ranges from 5% to 35%, depending on treatment protocol and study design. While recommendations exist for the repletion of coagulation factors with cryoprecipitate and fresh frozen plasma (FFP), there are no current consensus recommendations for the replacement of AT in patients receiving asparaginase. This study aims to assess if administration of AT replacement products decreased the risk of thrombosis in ALL, lymphoblastic lymphoma, and NK/T-cell lymphoma patients with asparaginase-induced deficiency. Secondarily, this study investigated the risk of hemorrhage, budgetary impact of AT replacement products, and survival in this patient population.
Methods
This retrospective study evaluated patients 18 years of age or older who received L-asparaginase, pegaspargase, or asparaginase Erwinia chrysanthemi between January 2011 and June 2018 for ALL, lymphoblastic lymphoma, or NK/T-cell lymphoma. At our institution, a change in practice occurred in 2014 which led to the monitoring and supplementation of AT in this patient population. AT levels were monitored in patients post asparaginase therapy and supplementation was provided when levels were below 60%. Patients receiving anticoagulation treatment prior to the start of asparaginase therapy were excluded from the study. Data were analyzed using Chi-squared and T-tests were used to assess for significance between the groups.
Results
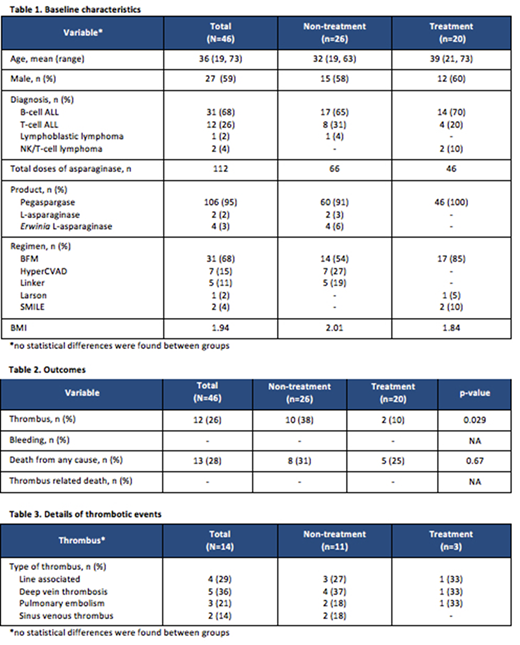
Forty-six patients were analyzed in total; the mean age was 36 years of age and the most common diagnoses were B-cell ALL and T-cell ALL; 26 patients did not receive AT monitoring while 20 patients were monitored and received AT repletion (Table 1). There was a statistically significant difference in the development of thrombi between the groups with only 2 patients (10%) in the treatment group developing a thrombus compared to 10 patients (38%) in the non-treatment group (p=0.029) (Table 2). Two patients in the non-treatment group developed a sinus venous thrombus. It should be noted, a patient in each group developed multiple thrombi (Table 3). Overall, no patients experienced a clinically significant bleeding event that required clinical intervention. Overall survival was similar between groups with death from any cause occurring in 5 patients (25%) in the treatment group and 8 patients (31%) in the non-treatment group (p=0.67) (Table 2). The average cost of AT replacement for patients receiving supplementation was $78,370.
Conclusion
The monitoring and supplementation of AT in ALL, lymphoblastic lymphoma, and NK/T-cell lymphoma patients was associated with a significant decrease in the occurrence of clinically significant thrombi. Patients in both groups did not experience bleeding events and the overall survival rate was similar between groups. While the average cost of AT repletion was considerable, the cost associated with a thrombus is arguably more substantial when taking into account intensive care admissions, diagnostic exams, and anticoagulation medications. Therefore, the result of this study suggests utilization of monitoring AT activity and its supplementation in patients receiving asparaginase therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal